. 2009. “Surgical applications of femtosecond lasers.” J. Biophoton., 2, Pp. 557–572. Publisher's VersionAbstract
Nanosurgery with femtosecond lasers
I. Zaharieva Maxwell. 2006. “Application of femtosecond lasers for subcellular nanosurgery”. Publisher's VersionAbstract
This dissertation o ers a study of femtosecond laser disruption in single cells. Cells and tissues do not ordinarily absorb light in the near-IR wavelength range of femtosecond lasers. However, the peak intensity of a femtosecond laser pulse is very high and material disruption is possible through nonlinear absorption and plasma generation. Because the pulse duration is very short, it is possible to reach the intensity of optical breakdown at only nanojoules of energy per pulse. The low energy deposition and the high spatial localization of the nonlinear absorption, make femtosecond laser pulses an ideal tool for minimally disruptive subcellular nanosurgery. We show definitively that there can be bulk ablation within a single cell by studying the disrupted region under a transmission electron microscope. The width of the ablated area can be as small as 250 nm in diameter at energies near the ablation threshold. We also studied the e ect of the laser repetition rate on the subcellular disruption threshold. We compared the pulse energies for kHz and MHz pulse trains, and found that in the MHz regime heat accumulation in the focal volume needs to be accounted for. For this repetition rate the minimum pulse energy necessary for disruption depends on the laser irradiation time. We used femtosecond laser nanosurgery to probe tension in actin stress fibers in living endothelial cells. By severing an individual stress fiber and visualizing its retraction, we showed that actin carries prestress in adherent, non-contractile cells. By plating the cells on softer, more compliant substrates, we measured the deflection of the substrate and extrapolated the force contribution of a stress filament on total amount of force exerted by the cell.
S. H. Chung, A. Schmalz, R. Clarissa Ruiz, C. V. Gabel, and E. Mazur. 2013. “Femtosecond Laser Ablation Reveals Antagonistic Sensory and Neuroendocrine Signaling that Underlie C. elegans Behavior and Development.” Cell Reports, 4, Pp. 316–326. Publisher's VersionAbstract
The specific roles of neuronal subcellular compo- nents in behavior and development remain largely unknown, even though advances in molecular biology and conventional whole-cell laser ablation have greatly accelerated the identification of contrib- utors at the molecular and cellular levels. We system- atically applied femtosecond laser ablation, which has submicrometer resolution in vivo, to dissect the cell bodies, dendrites, or axons of a sensory neuron (ASJ) in Caenorhabditis elegans to determine their roles in modulating locomotion and the develop- mental decisions for dauer, a facultative, stress- resistant life stage. Our results indicate that the cell body sends out axonally mediated and hormonal sig- nals in order to mediate these functions. Further- more, our results suggest that antagonistic sensory dendritic signals primarily drive and switch polarity between the decisions to enter and exit dauer. Thus, the improved resolution of femtosecond laser ablation reveals a rich complexity of neuronal signaling at the subcellular level, including multiple neurite and hormonally mediated pathways depen- dent on life stage.
N. Shen, D. Datta, C. B. Schaffer, P. LeDuc, D. E. Ingber, and E. Mazur. 2005. “Ablation of cytoskeletal filaments and mitochondria in cells using a femtosecond laser nanoscissor.” Mechanics and Chemistry of Biosystems, 2, Pp. 17–26. Publisher's VersionAbstract
Analysis of cell regulation requires methods for perturbing molecular processes within living cells with spatial discrimination on the nanometer- scale. We present a technique for ablating molecular structures in living cells using low-repetition rate, low-energy femtosecond laser pulses. By tightly focusing these pulses beneath the cell membrane, we ablate cellular material inside the cell through nonlinear processes. We selectively removed sub- micrometer regions of the cytoskeleton and individual mitochondria without altering neighboring structures or compromising cell viability. This nanoscissor technique enables non-invasive manipulation of the structural machinery of living cells with several-hundred-nanometer resolution. Using this approach, we unequivocally demonstrate that mitochondria are structurally independent functional units, and do not form a continuous network as suggested by some past studies. Keywords: Nanoscissor; nanosurgery; femtosecond laser; photodisruption; cytoskeleton; mitochondria
A. Heisterkamp, I. Zaharieva Maxwell, E. Mazur, J. M. Underwood, J. A. Nickerson, S. Kumar, and D. E. Ingber. 2005. “Pulse energy dependence of subcellular dissection by femtosecond laser pulses.” Opt. Express, 13, Pp. 3690–3696. Publisher's VersionAbstract
Precise dissection of cells with ultrashort laser pulses requires a clear understanding of how the onset and extent of ablation (i.e., the removal of material) depends on pulse energy. We carried out a systematic study of the energy dependence of the plasma-mediated ablation of fluorescently-labeled subcellular structures in the cytoskeleton and nuclei of fixed endothelial cells using femtosecond, near-infrared laser pulses focused through a high- numerical aperture objective lens (1.4 NA). We find that the energy threshold for photobleaching lies between 0.9 and 1.7 nJ. By comparing the changes in fluorescence with the actual material loss determined by electron microscopy, we find that the threshold for true material ablation is about 20% higher than the photobleaching threshold. This information makes it possible to use the fluorescence to determine the onset of true material ablation without resorting to electron microscopy. We confirm the precision of this technique by severing a single microtubule without disrupting the neighboring microtubules, less than 1 m away.
M. Zhang, S. H. Chung, C. Fang-Yen, C. Craig, R. A. Kerr, H. Suzuki, A. D. T. Samuel, E. Mazur, and W. R. Schafer. 2008. “A self-regulating feed-forward circuit controlling C. elegans egg- laying behavior.” Curr. Biol., 18, Pp. 1445–1455. Publisher's VersionAbstract
Background Egg laying in Caenorhabditis elegans has been well studied at the genetic and behavioral levels. However, the neural basis of egg-laying behavior is still not well understood; in particular, the roles of specific neurons and the functional nature of the synaptic connections in the egg- laying circuit remain uncharacterized. Results We have used in vivo neuroimaging and laser surgery to address these questions in intact, behaving animals. We have found that the HSN neurons play a central role in driving egg-laying behavior through direct excitation of the vulval muscles and VC motor neurons. The VC neurons play a dual role in the egg-laying circuit, exciting the vulval muscles while feedback-inhibiting the HSNs. Interestingly, the HSNs are active in the absence of synaptic input, suggesting that egg laying may be controlled through modulation of autonomous HSN activity. Indeed, body touch appears to inhibit egg laying, in part by interfering with HSN calcium oscillations. Conclusions The egg-laying motor circuit comprises a simple three-component system combining feed-forward excitation and feedback inhibition. This microcircuit motif is common in the C. elegans nervous system, as well as in the mammalian cortex; thus, understanding its functional properties in C. elegans may provide insight into its computational role in more complex brains.
A. Heisterkamp, J. Baumgart, I. Zaharieva Maxwell, A. Ngezahayo, E. Mazur, and H. Lubatschowski. 2007. “Fs-Laser Scissors for Photobleaching, Ablation in Fixed Samples and Living Cells, and Studies of Cell Mechanics.” In Laser Manipulation of Cells and Tissues, edited by Michael Berns and Karl Greulich, Pp. 293–307. Academic Press. Publisher's VersionAbstract
The use of ultrashort laser pulses for microscopy has steadily increased over the past years. In this so-called multiphoton microscopy, laser pulses with pulse duration around 100 femtoseconds (fs) are used to excite fluorescence within the samples. Due to the high peak powers of fs lasers, the absorption mechanism of the laser light is based on nonlinear absorption. Therefore, the fluorescence signal is highly localized within the bulk of biological materials, similar to a confocal microscope. However, this nonlinear absorption mechanism can not only be used for imaging but for selective alteration of the material at the laser focus: The absorption can on one hand lead to the excitation of fluorescent molecules of fluorescently tagged cells by the simultaneous absorption of two or three photons or on the other hand, in case of higher order processes, to the creation of free-electron plasmas and, consequently, plasma-mediated ablation. Typical imaging powers are in the range of tens of milliwatts using 100-fs pulses at a repetition rate of 80-90 MHz, while pulse energies needed for ablation powers are as low as a few nanojoules when using high numerical aperture microscope objectives for focusing the laser radiation into the sample. Since the first demonstration of this technique, numerous applications of fs lasers have emerged within the field of cellular biology and microscopy. As the typical wavelengths of ultrashort laser systems lie in the near infrared between 800 and 1000 nm, high penetration depth can be achieved and can provide the possibility of imaging and manipulating the biological samples with one single laser system.
S. H. Chung, D. A. Clark, C. V. Gabel, E. Mazur, and A. Samuel. 2006. “The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation.” BMC Neuroscience, 7, Pp. 30–. Publisher's VersionAbstract
Background: Caenorhabditis elegans actively crawls down thermal gradients until it reaches the temperature of its cultivation, exhibiting what is called cryophilic movement. Implicit in the worms ability to actively bias its movements down thermal gradients is an ability to detect thermal gradients, and implicit in regulating the display of cryophilic bias is the ability to compare current ambient temperature with a stored memory of cultivation temperature. Several lines of evidence link the AFD sensory neuron to thermotactic behavior, but its exact role is not yet known. A current model contends that AFD is part of a thermophilic mechanism which biases movement up thermal gradients that counterbalances a cryophilic mechanism which biases movement down thermal gradients. Results: We used tightly-focused femtosecond laser pulses to dissect the AFD neuronal cell bodies and the AFD sensory dendrites in C. elegans to investigate their contribution to biased cryophilic movement. We establish that femtosecond laser ablation can exhibit submicrometer precision allowing the severing of individual AFD nerve fibers without causing collateral damage. Severing AFD dendrites in young adult worms permanently abolishes their sensory contribution without functional regeneration. We show that thermosensory input to the AFD neuron is required to activate a mechanism for generating cryophilic bias, but we find no evidence that AFD laser surgery reduces a putative ability to generate thermophilic bias. In addition, although disruption of the AIY interneuron causes worms to exhibit cryophilic bias at all temperatures, we find no evidence that disruption of the AIZ interneuron causes thermophilic bias at any temperature. Conclusions: We conclude that laser surgical analysis of the thermotactic circuit does not support a current model in which AFD opposes cryophilic bias by generating thermophilic bias. Our data supports a model in which a mechanism for generating cryophilic bias is gated by the AFD neurons.
I. Zaharieva Maxwell, S. H. Chung, and E. Mazur. 2005. “Nanoprocessing of subcellular targets using femtosecond laser pulses.” Med. Laser Appl., 20, Pp. 193–200. Publisher's VersionAbstract
In this paper we review the work done in our laboratory on femtosecond laser dissection within single cells and living organisms. Precise dissection of biological material with ultrashort laser pulses requires a clear understanding of the pulse-energy dependence of the onset and extent of plasma-mediated ablation (i.e., the removal of material). We carried out a systematic study of the energy dependence of the plasma-mediated ablation of fluorescently-labeled subcellular structures in the cytoskeleton and in nuclei of fixed endothelial cells using femtosecond, near- infrared laser pulses focused through a high- numerical aperture objective lens (1.4 NA). We performed laser nanosurgery in live cells, where we ablated a single mitochondrion and severed cytoskeletal filaments without compromising the cell membrane or the cells viability. We also cut dendrites in living C. elegans without affecting the neighboring neurons. This nanoprocessing technique enables non-invasive manipulation of the structural machinery of cells and tissues down to several-hundred- nanometer resolution.
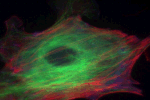 The detailed architecture of spindle microtubules, involved in cell division, was revealed using femtosecond laser nanosurgery. This work was performed in collaboration with the Needleman group (results published in Cell).
The detailed architecture of spindle microtubules, involved in cell division, was revealed using femtosecond laser nanosurgery. This work was performed in collaboration with the Needleman group (results published in Cell).